4.2 Corrosion and erosion in pumps
Corrosion and erosion in pumps
Corrosion and erosion in pumps is largely an electrochemical process, i.e. there is a positive electrode, the anode, and a negative cathode, between which an electric current will flow. If an iron component is immersed in acidic water, the anode-cathode process will occur over its entire surface. The cathode in this case comprises local elements of more noble metals in the galvanic series segregated in the alloying process, or non-metallic inclusions or by local reaction with oxides, sulphides and so on. Metal is dissolved by the generation of metal ions at the anode. Oxygen dissolved in the liquid is reduced by the generation of hydroxyl ions at the cathode, or in the absence of oxygen hydrogen ions H+ are used up in the generation of hydrogen gas (Other chemical reactions occur at the cathode with certain other liquid metal combinations). Where metallic and hydroxyl ions come into contact, corrosion products are generated, usually in several stages of chemical reaction. The final corrosion product usually comprises metal oxides, which are difficult to dissolve, or metal hydroxides. Corrosion products generate a more or less dense film on the surface.
The incidence of protective films resulting from corrosion, or those generated in other ways, are necessary as a rule for most metals in use in order that further corrosion should be inhibited, or contained within reasonable limits. Only exclusive noble metals like gold and platinum are immune within the greater part of the stable region of water.
Chemical reactions increase exponentially with temperature. The chemical rule of thumb that a rate of reaction doubles with a temperature increases of 10°C also applies to the corrosion process. As temperature increases, however, secondary effects can come into play, resulting in reduced corrosion. The oxygen content of water at atmospheric pressure decreases as temperature increases, thus reducing the corrosive effect on, for example, iron. If there is positive pressure, dissolved gases such as O2 and H2 will be present in the water even at high temperatures and will still influence corrosion.
When a centrifugal pump drives against a closed valve, the required motor power will be converted to heat in the pump, which rapidly causes high temperatures. Especially in the case of corrosive liquids, this can lead to heavy attacks because a passive film can be destroyed. Heating can also mean mechanical damage resulting from too high a liquid pressure or deteriorating material strength. Plastic pumps are especially prone to softening with heat and should therefore be fitted with temperature sensors.
Rate of corrosion
For a material to be considered in practice as being completely resistive, the material lost from the surface should not exceed about 0.1 mm per year. If the material losses are of the order of magnitude of 1 mm per year, the material can still be used but must be closely supervised, checked and replaced at certain intervals.
Corrosion rates are expressed either as penetration depths or loss of weight per unit area per unit time. The conversion factors for some commonly used units are tabulated in figure 4.2a.
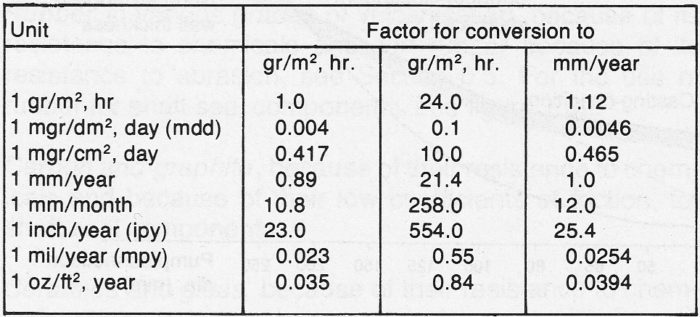
Figure 4.2a Conversion of various units for rate of corrosion 1 mil = 0.001 inch. For depth of penetration, the conversion applies only to steel or substances of the same density.
Types of corrosion
Units for rate of corrosion assume an even attrition over the whole surface, i.e. uniform corrosion. This often does not happen and other forms of corrosion can occur:
- Electrolytic (galvanic) corrosion, which occurs when different metals come into contact.
- Crevice corrosion, which occurs in crevices, where quantities of liquid may become more or less isolated from the main body of the liquid.
- Pitting, meaning that the corrosion is concentrated at certain points, pits.
- Inter-granular (inter-crystalline) corrosion usually associated with redistribution of alloying elements during heat treatment.
- Selective corrosion, where one single alloying element is dissolved out, e.g. zinc fall-out in brass.
- Erosion-corrosion, which occurs in flowing media and is the commonest complication in pumps.
- Stress-corrosion, where a combination of mechanical stress and attack by the liquid may lead to fracture.
Crevice corrosion and pitting are related phenomena, and are most troublesome for stainless steel in a chloride containing environment. These attacks occur in crevices or holes where the conditions for setting up a protective film are absent because of screening from the surrounding solution. Special care must be taken in pumps to prevent crevice corrosion at rotor fixings or on shafts rotating within seals. The risk of crevice corrosion is reduced in flowing liquids. Crevice corrosion will not usually occur so long as the pump is working. During longish stationary periods in chloride-containing liquids, however, the pump should be emptied and swilled out with water.
So-called corrosion fatigue is also of interest in pumps and especially for shafts. Even seemingly insignificant corrosion may reduce the fatigue strength of a material by a factor of 3 to 10.
Galvanic (electrolytic) corrosion
Where there is a combination of different metals, galvanic corrosion may occur. This means that the less noble metal, the anode, has a higher corrosion rate than the more noble metal, the cathode, which is protected instead. An indication of the risks of galvanic corrosion can be obtained by studying the so-called galvanic (electrolytic) series, see figure 4.2b. In practice, metals with a potential difference of 0.2V (volt) can be connected together without any trouble. Galvanic corrosion is of great significance in pumps because of the principle of using different materials for various parts. In practice, however, it is apparent that the effects of erosion-corrosion motivate relatively wide departures from good practice from the galvanic corrosion viewpoint.
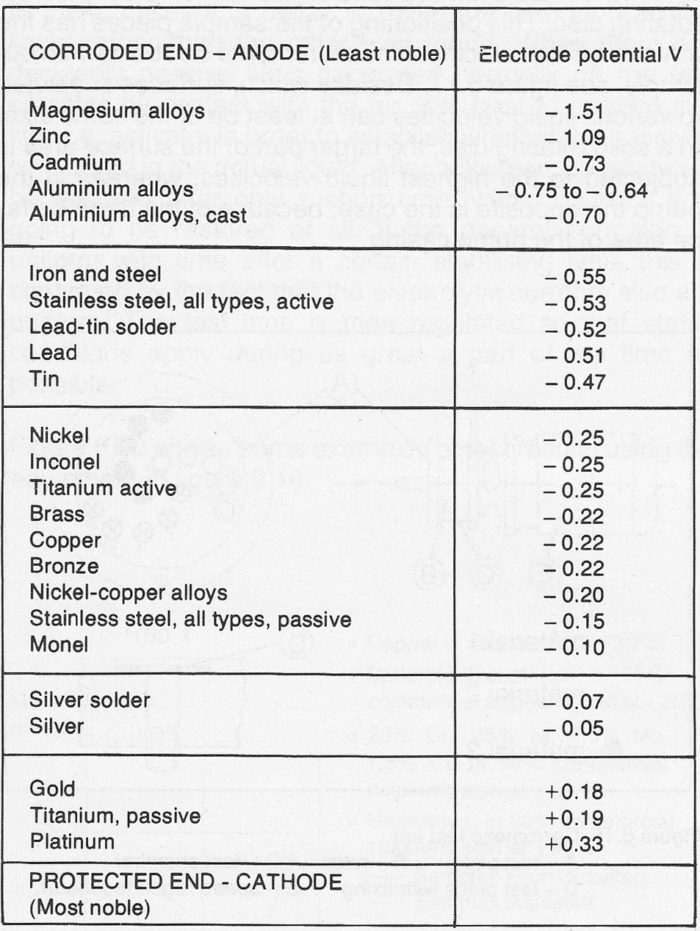
Figure 4.2b Galvanic series for metals with potential values measured in sea-water (source, S. Bartha)
Where the anode surfaces are large and cathode surface is small, in the case of pump casings and impellers, for example, the potential differences for certain favorable combinations can be twice as much. Figure 4.2c shows a drastic case of galvanic corrosion.

Figure 4.2c Galvanic corrosion on type 13 Cr stainless steel pump shaft. The shaft has been lying in stock fitted with graphite containing box seals, the being caused by condensation from humid atmosphere. The attack has been hastened by crevice corrosion.
Erosion-corrosion
Erosion-corrosion is particularly common in centrifugal pumps where the flow velocity nearly always exceeds 20 m/s, against 1 to 2 m/s in pipelines. Characteristic of erosion-corrosion are the horseshoe-shaped patterns undercut in the direction of flow and bright surfaces free of corrosion products, see figure 4.2d.
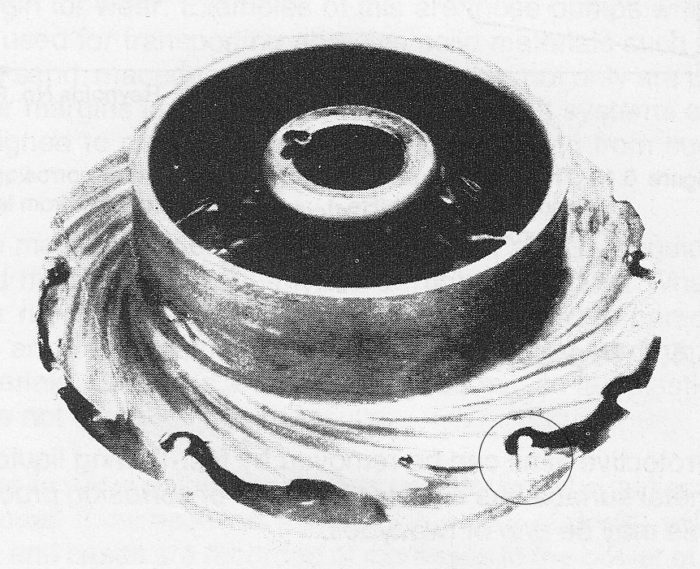
Figure 4.2d Erosion-corrosion on a type 13 Cr + Mo pump impeller used for sulphate liquor. Note that the rivets are completely intact. These were made of Stainless Steel 316L. For this application the rotor should have been made of 316L.
When metal comes under attack, oxidisation media O2 or H+ for example, are consumed at the surface of the metal and metal ions are generated. Thus the concentration of metal ions will be greater and the concentration of oxidisation media will be lower at the metal surface than in the main body of the liquid, see figure 4.2d. The difference in concentration at the metal surfaces is dependent upon the difficulties of transportation of the participating substances which is necessary for the continuation of the corrosion process.
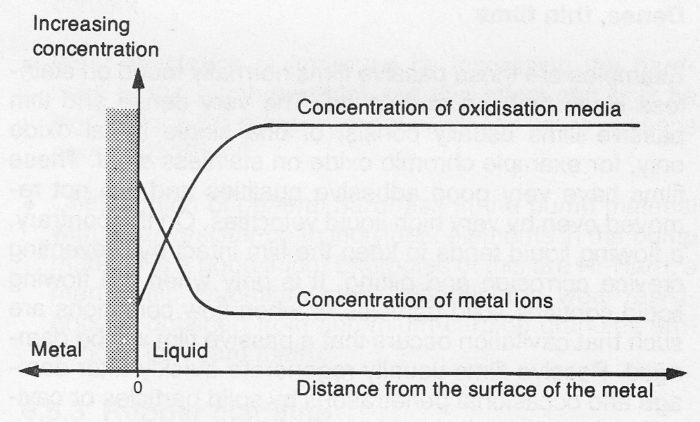
Figure 4.2e Concentration differences of general corrosion at a metal surface.
Obviously the transport will be aided and, at the same time, the rate of corrosion will be accelerated if the liquid is in motion, which is generally the case in centrifugal pumps, see figure 4.2e. Care should thus be taken when using corrosion data from tests in stationary liquids for the selection of pump materials, since corrosive attack inside a pump may be ten times worse than it is in a still liquid, see also figure 4.3c.
Protective films
Protective films plays an important role in protecting from corrosion and erosion in pumps but they can be removed by fast-flowing liquid. If a metal surface has a protective layer of corrosion products, this may be one of two types:
Porous, relatively thick films
Examples are the film on copper alloys and graphite on cast iron. Thick protective films often have complex constructions, because contaminants in the liquid, such as lime, may be included as a significant component. It is for this reason, difficult to predict whether any effective film will be generated at all. At speeds normally found in centrifugal pumps, these films often have an abrupt limit above which they get carried away by the flow of liquid. Thick films are relatively sensitive to solid particles, cavitation and even air bubbles in the flowing liquid. Even if such disturbance is sporadic, the damage may be great because the films take a relatively long time to regenerate, days or weeks even.
Dense, thin films
Examples are those passive films normally found on stainless steel, titanium and so on. The very dense and thin passive films usually consist of one single metal oxide only, for example chromic oxide on stainless steel. These films have very good adhesive qualities and are not removed even by very high liquid velocities. On the contrary, a flowing liquid tends to keep the film intact by preventing crevice corrosion and pitting. It is only when the flowing liquid contains solid particles or when flow conditions are such that cavitation occurs that a passive film will be damaged. Passive films usually regenerate quickly after damage and occasional penetrations by solid particles or cavitation do not usually lead to further corrosion. If, for any reason, a passive film does not exist on stainless steel, titanium and so on, then heavy corrosion attack can be expected.
