7.1 Water properties
Water properties for pump applications
Water properties for pump applications describe the different aspects one need to consider to select a proper pump when pumping water. Pumping water is normally done with a centrifugal pump but to select the proper material, design and motor size, there are a number of properties of the water that needs to be specified.
Fresh water
By fresh water we generally mean naturally non-salty water within the temperature range of 0-30°C occurring in nature as surface water in lakes and rivers and also occurring as ground water. Both spring and tap water also belong here. Natural water has enormous usage, among other things as raw water for drinking purposes, pumping water is also a necessity as cooling and process water within various industries and for irrigation.
For the purposes of pumping installations, pumping water, the most interesting aspect is the corrosive action of water on the commonly used construction materials, generally steel, grey cast iron and bronze. The characteristics of water which contribute to corrosion are:
- pH value
- hardness and carbonic acid content
- content of various chemicals, primarily salts
- acidity
Apart from these, the temperature of the water and the following factors will affect the installation.
- velocity of flow; normally several m/s in pipes and 10-40 m/s in a centrifugal pump
- cavitation, both fully and partially generated
- content of solid bodies, e.g. sand and sludge from various sources.
As a rule, pump installation factors show a strong negative reaction when the properties of the water are unfavorable or barely favorable. It would be very difficult here to make general rules when even an insignificant quantity of salt may magnify an attack of corrosion. Water containing chlorides can be especially troublesome. The corrosive action on steel, for example, is increased by a factor of 8 at 50 mg MgCl2/litre, which means that steel is not practicable for this purpose.
pH value and choice of material in a pump
Natural waters usually have pH values of between 4 and 8. They are divided into two main groups according to their acid content.
- Ground water from deep down: this contains very little acid and it is thus the hydrogen ion concentration which is the decisive factor in the aggressiveness of the water. It should be pointed out that iron is attacked noticeably at pH values of 6— 7 in this low acid water.
- Surface water, which is acidic: here the pH value is no absolute measure of aggressiveness although it is important to know what it is.
Mains water may be low acid ground water or high acid surface water which has, furthermore, been treated chemically. Waterworks supply the necessary information in this respect. Special conditions apply for lime-containing water, which is dealt with in the following section.
Since pumps used for conveying fresh water are for the most part constructed of cast-iron, some general aspects are given below about the use of this material with particular reference to the pH value of the water.
- Grey cast iron may be used without any real problems within the pH range 7-10. If chlorides are present, it may be that cast iron is not adequate.
- Grey cast iron can often be used within the pH range 5-7, but the effects of those factors arising from the lower pH values can be great. Within this pH range, cast iron is superior to steel as regards resistance to corrosion. The high carbon content of grey cast-iron (3 to 4%) means that at moderate speeds of flow the graphite, together with corrosion products, can build up an anti-corrosive film, so-called graphitization.
For pH values at which cast-iron is not resistent, bronze or stainless steel has to be used. (See also section about Materials for pumps). It should be emphasized that the pH value of water can always be adjusted by suitable chemical treatment before pumping.
Chemically pure water
The hydraulic properties of chemically pure, i.e. distilled water can be determined with very great accuracy. The viscosity and vapor (saturation) pressures given below conform to the international standard values accepted.
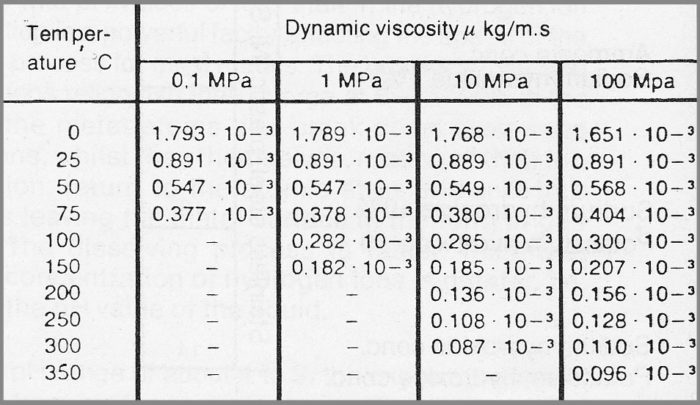
Figure 7.1a Dynamic viscosity of chemically pure water at various temperatures and pressures.

Figure 7.1b Dynamic viscosity, kinematic viscosity and density for chemically pure water in the temperature range -25°C to 99°C and at a pressure of 0.1 MPa (1 bar).

Figure 7.1c Density of chemically pure water at various temperatures and pressures

Figure 7.1d Vapor (saturation) pressure for chemically pure water.
Various characteristics of chemically pure water:
- Melting point, ice to water, 0°C at 0.1 MPa (1 bar)
- Latent heat of fusion, ice to water, 335 kJ/kg
- Specific heat of chemically pure water 4.182 kJ/kg, K at 0.1 MPa and 20°C
- Boiling 100°C at 0.1 MPa
- Latent heat to steam 2,260 kJ/kg at 100°C
- Coefficient of expansion γ = 0.207 x 10‾³ 1/°C at 0.1 MPa and 20°C
- Bulk modulus K = 2.2 x 10‾9 N/m² at 0.1 MPa and 20°C
Hardness of water
The degree of hardness of water depends upon the presence of impurities, mainly calcium (Ca) and magnesium (Mg) in the form of carbonates, although non-carbonates, for example sulphates, nitrates and chlorides, also have an effect. The quantities in which these impurities occur are a measure of the hardness of the water. Hardness can be expressed in terms of the specific substance such as calcium hardness (Ca-H), magnesium hardness (Mg-H) and so on. The total hardness comprises the sum of the individual hardness. Distinction can also be made between permanent and temporary hardness. Temporary hardness consists of alkali ions bound to carbonates, and permanent hardness of alkali ions bound to non-carbonates. Temporary hardness is so called because it disappears with heating. Distribution under the various headings is according to the chemically equivalent content of alkali ions. Since the various alkaline metals have different atomic weights, the unit for hardness, 1 milli–equivalent per litre (meq/l) is defined as:
1 meq/l = (1 m mol/l ) / (chemical valency)
The hardness unit 1 meq/litre corresponds to the following ion contents in mg/l :
- 1 meq calcium hardness = 20.04 mg/l Ca++
- 1 meq magnesium hardness = 12.16 mg/l Mg++
- 1 meq strontium hardness = 43.82 mg/l Sr++
- 1 meq barium hardness = 68.68 mg/l Ba++
Other units to express hardness of water, in terms of concentration, are used in other countries. For conversion of various hardness units, see figure 1.13.
Figure 1.13 Conversion factors for various degrees of hardness
